17
2023
-
11
The 3rd CIBM Biometrics Development Conference and the 2nd Biological Standards Technology Exchange Conference were successfully held in Zhengzhou, Henan Province
On November 7, the 3rd CIBM Biometrics Development Conference and the 2nd Biological Standards Technology Exchange Conference were grandly opened in Zhengzhou City, Henan Province. The conference attracted many academicians, experts, government managers and entrepreneurs from the fields of biometrics, standardization, life sciences, biological industry and other fields to carry out in-depth exchanges of policy, research, academic and industry. The theme of the conference report is closely focused on the high-quality development of biotechnology and bioeconomy, which is in line with the goal of China's modernization.
On November 7, the 3rd CIBM Biometrics Development Conference and the 2nd Biological Standards Technology Exchange Conference were grandly opened in Zhengzhou City, Henan Province. The conference attracted many academicians, experts, government managers and entrepreneurs from the fields of biometrics, standardization, life sciences, biological industry and other fields to carry out in-depth exchanges of policy, research, academic and industry. The theme of the conference report is closely focused on the high-quality development of biotechnology and bioeconomy, which is in line with the goal of China's modernization.
This year is the 20th year of China's biometric development from its inception. This conference extends the purpose of CIBM's "quality, safety and health"-"biometric accurate traceability, build a solid quality foundation to ensure health and safety". The main purpose of this conference is to promote the cross-integration of various fields and promote the continuous progress of biometrics and standards, with the goal of promoting the high-quality development of biotechnology and bioeconomy, ensuring people's health and safety, and ecological security, and making positive contributions to the realization of Chinese-style modernization.
Biometrics, as an important support for the development of biotechnology and industry, carries a major mission. In today's world, biometrics workers have a major responsibility. They are committed to ensuring quality, safety and health in life sciences, healthcare, and agricultural production. The conference provides a platform for experts from all walks of life to exchange ideas and share experiences, and jointly contribute wisdom and strength to promote the progress of China's biometrics field and achieve national development goals. It also injects new vitality into the modernization of China's biometrics field and brings new opportunities.
In this conference, Li Yang, founder and CEO of Finowicom, was invited to give a thematic report on "Application of Intelligent External Quality Evaluation in Histopathological Detection" at the "Innovation of Biological Reagents and Biological Instruments.
Quality control is the basis of clinical diagnosis, and a perfect quality control system can promote the mutual recognition of inspection results among medical institutions. The premise of mutual recognition of test results is to establish an effective laboratory quality control system. At the same time, as an analytical instrument or method performance material, quality control products came into being to provide guarantee for effective monitoring of experimental quality.
In the routine pathological examination, due to the lack of sustainable regeneration of immunohistochemical standards, resulting in a significant increase in medical costs. Compared with immunohistochemistry, the analytical error rate (<1%) in clinical chemistry, immunology, and hematology is an order of magnitude lower. In order to solve this problem, Finowicam has developed an international advanced cell quality control preparation process and a tissue pathology-oriented quality control system, which can provide industrial-grade quality control products for routine pathological tests, such as immunohistochemistry, in situ hybridization, PCR, etc., and has been used in the domestic market.
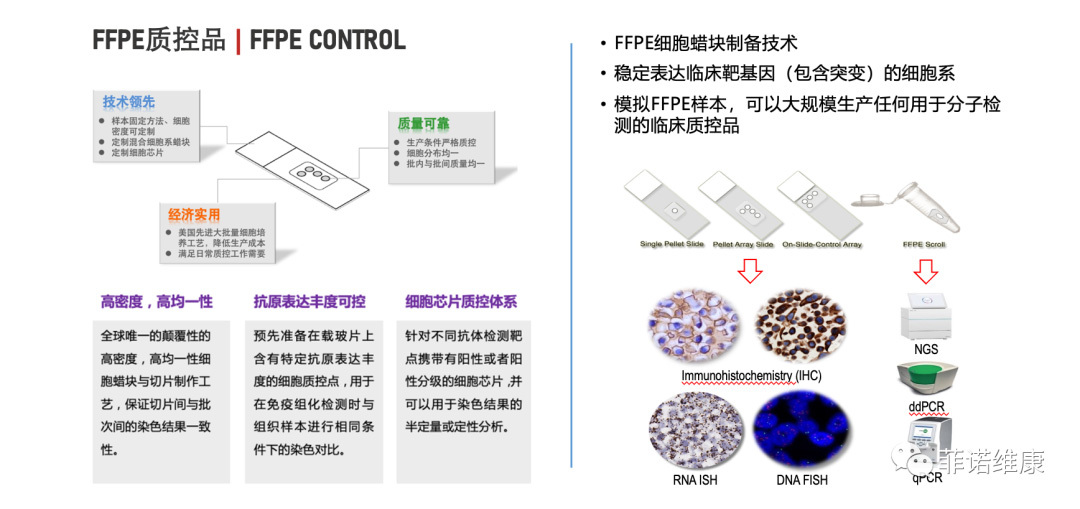
At the same time, by introducing the international advanced concept of external quality system control, Finowicam monitors the results of histopathological experiments by artificial intelligence, providing an effective guarantee for the stability of clinical pathology test results.
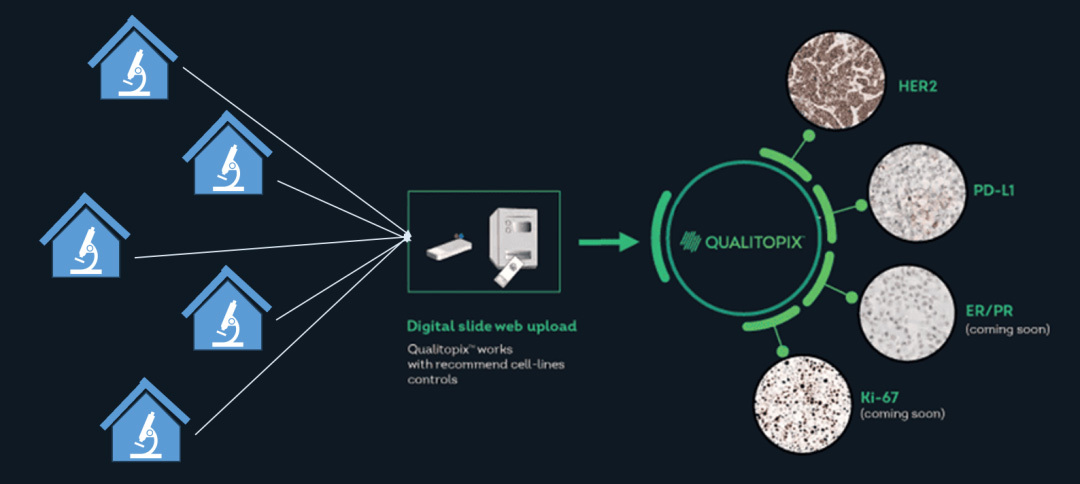
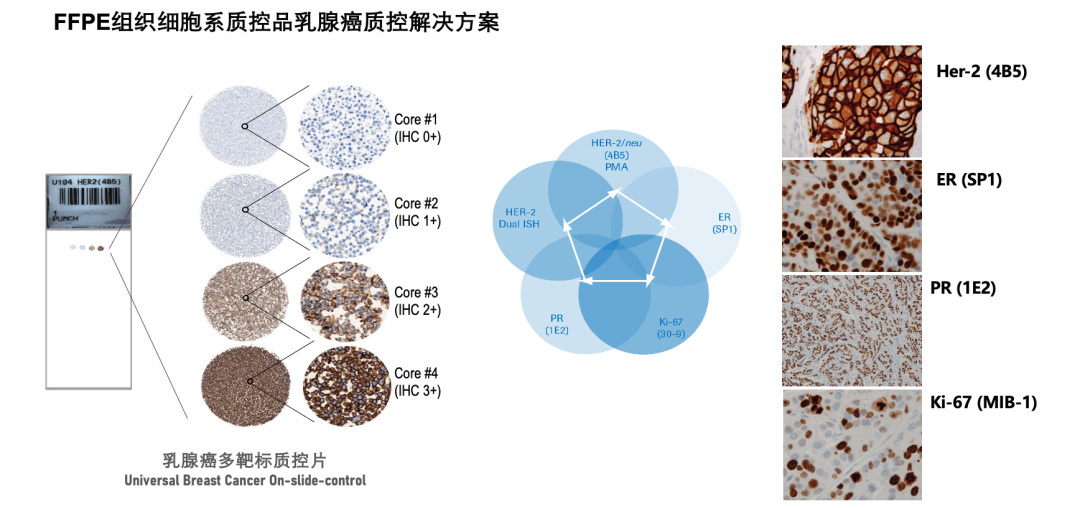
Finowicam is committed to providing spatial multi-omics integrated solutions to promote clinical translational medicine and new drug development progress. In the field of quality control has launched a number of product solutions, welcome to cooperate!
Key words:
Latest information












