Technical background
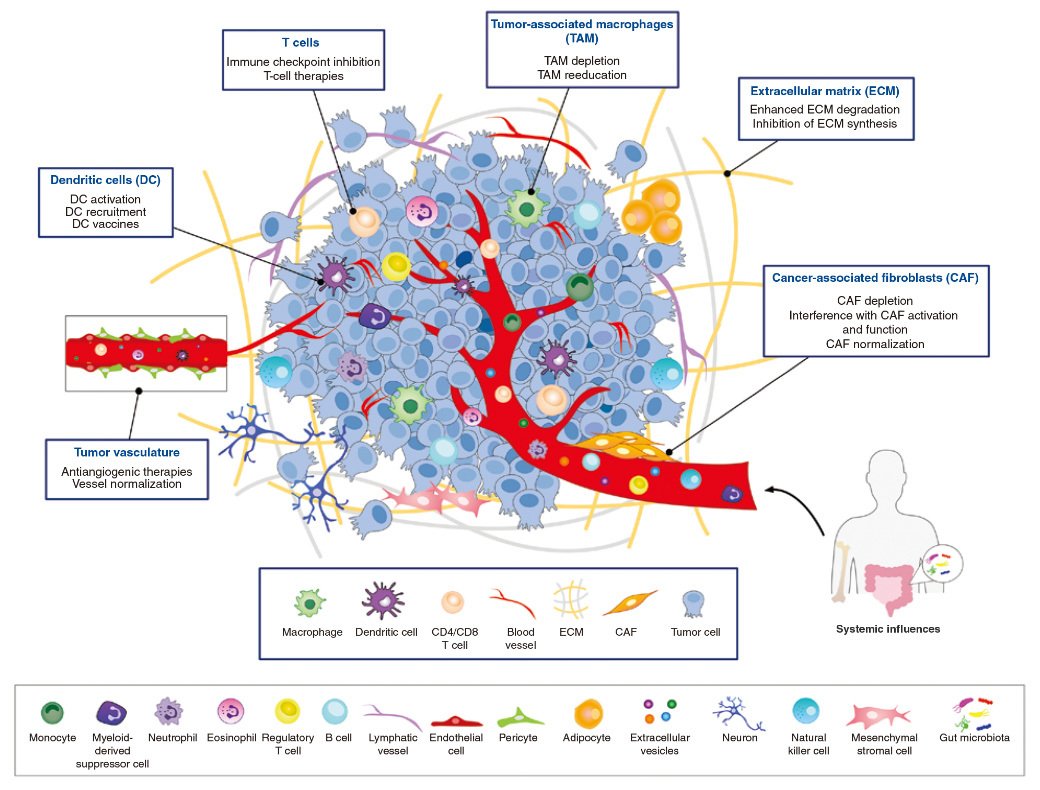
In 2022, spatial multi-omics technology was rated as one of the seven "subversive" technologies by Nature. It combines the detection results of genome, transcriptome, proteome and metabolome with the in situ information of tissues to reshape the positioning and distribution of relevant group information in the spatial three-dimensional structure. With the approval of several immunotherapy drugs, tumor immunotherapy is becoming more and more mature, and people pay more and more attention to the concept of tissue microenvironment and its role in tumor immunotherapy. Tumor cells can affect the surrounding microenvironment by releasing extracellular signals, promote tumor angiogenesis and inhibit the surrounding immune cells, and the immune cells and factors in the tumor microenvironment can affect the growth of tumor cells (see the figure below).
Service Content
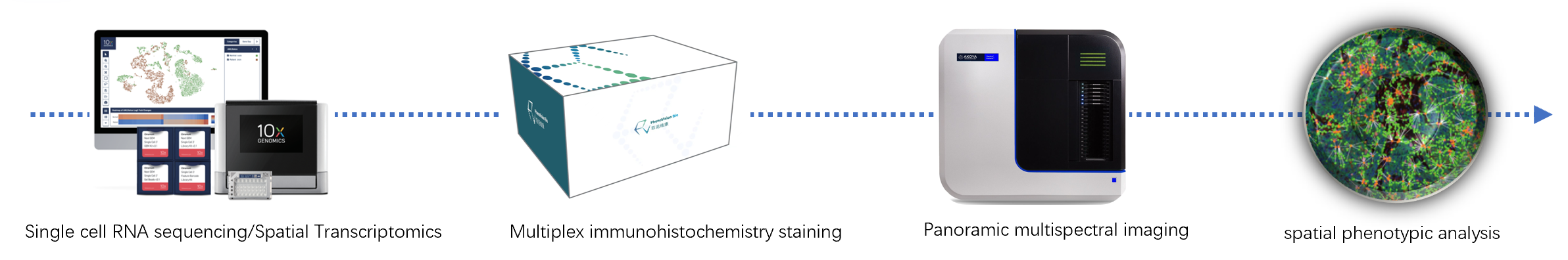
A wide range of spatial multi-omics technologies are available, from high-throughput spatial transcriptomics and proteomics to low-throughput RNA in situ detection techniques and multiplex fluorescence immunohistochemistry (IHC) methods. Each of these methodologies plays a unique role in its respective application area. PhenoVision Bio Co., Itd possesses an advanced spatial multi-omics platform and downstream data analysis platform. Backed by a team of experienced professionals with years of expertise in this field, PhenoVision integrates R&D, laboratory services, and clinical application development. It offers a one-stop spatial biology solution for multi-omics (DNA, RNA, and protein) analysis.
Direction of application
Case Show
Spatial phenotyping analysis can categorize marker panels into several major types: 1.Identifying the distribution of T cells and their subtypes (e.g., cytotoxic T cells, helper T cells) within tumors. 2.Distinguishing the distribution of M1 and M2 macrophages within tumors. 3.Focusing on different B cell subtypes (e.g., B cell subtypes represented by CD20, CD21) and the distribution of tertiary lymphoid structures within tumors. 4.Paying attention to a broader range of immune cell types, such as incorporating NK cells and dendritic cells into the panel to observe the distribution of different immune cells within tumors. 5.Focusing on combining tumor markers and immune checkpoints to observe the distribution of several major cell types (tumor cells, B cells, T cells, macrophages, etc.) within tumors.
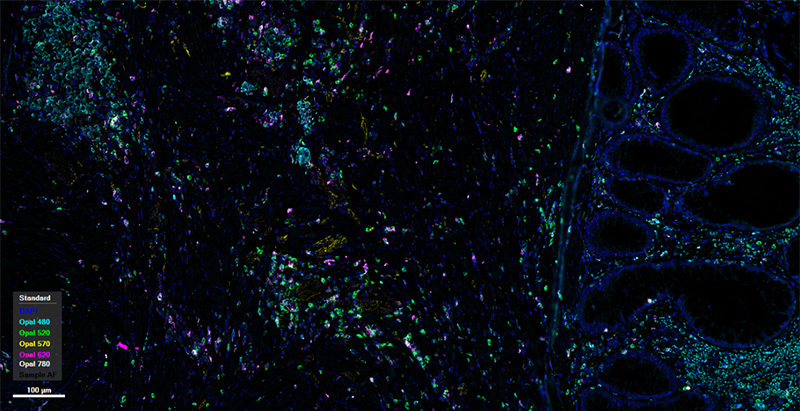
human intestinal cancer tissue
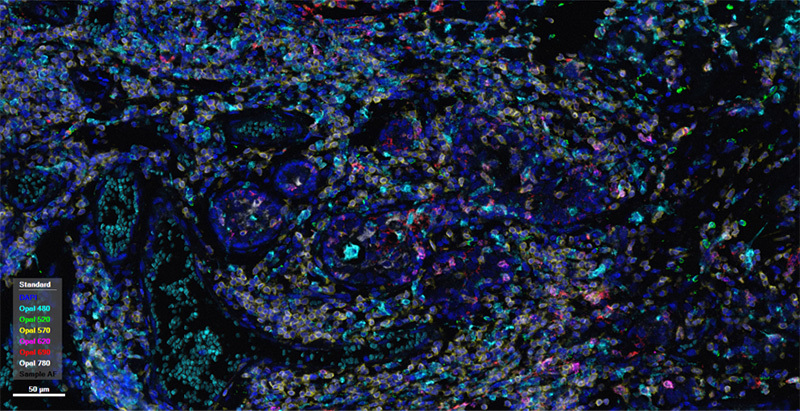
human breast cancer tissue
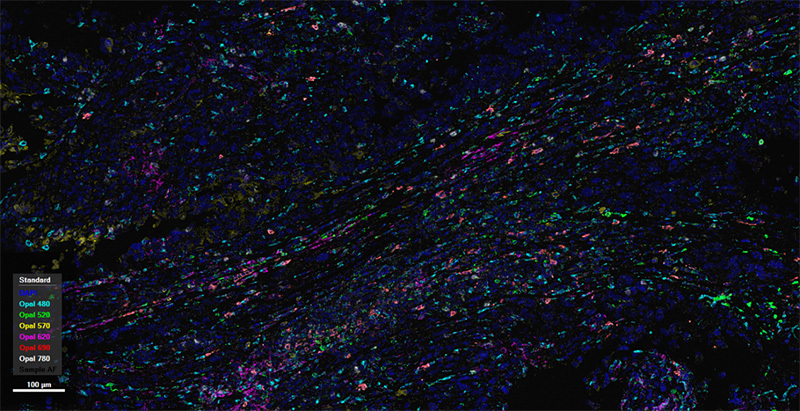
human melanoma tissue
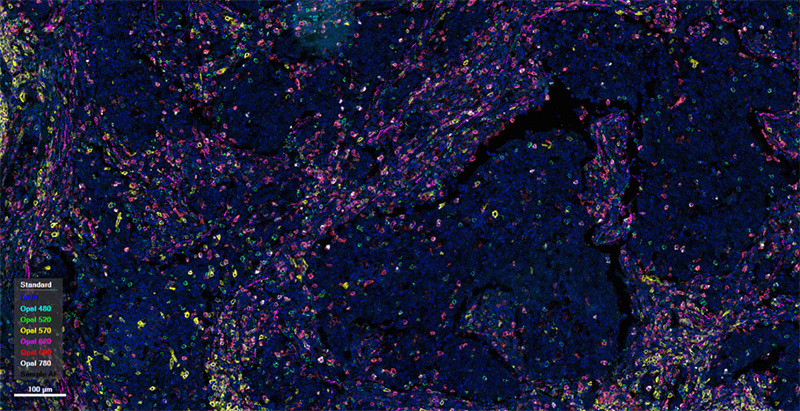
human lung cancer tissue
References
- Lu S, Stein JE, Rimm DL, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5(8):1195-1204. doi:10.1001/jamaoncol.2019.1549.
- Gao J, Navai N, Alhalabi O, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med. 2020 Dec;26(12):1845-1851. doi: 10.1038/s41591-020-1086-y. Epub 2020 Oct 12.
- Park, Y.H., Lal, S., Lee, J.E. et al. Chemotherapy induces dynamic immune responses in breast cancers that impact treatment outcome. Nat Commun. 2020 Dec 2;11(1):6175.doi: 10.1038/s41467-020-19933-0.
- Biswas S, Mandal G, Payne KK, et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature. 2021 Mar;591(7850):464-470. doi: 10.1038/s41586-020-03144-0. Epub 2021 Feb 3. PMID: 33536615.
- Berry S, Giraldo NA, Green BF, et al. Analysis of multispectral imaging with the AstroPath platform informs efficacy of PD-1 blockade. Science. 2021 Jun 11;372(6547):eaba2609. doi: 10.1126/science.aba2609. PMID: 34112666.
- Hoyt CC. Multiplex Immunofluorescence and Multispectral Imaging: Forming the Basis of a Clinical Test Platform for Immuno-Oncology. Front Mol Biosci. 2021 Jun 2;8:674747. doi: 10.3389/fmolb.2021.674747.
- Taube JM, Roman K, Engle EL, et al. Multi-institutional TSA-amplified Multiplexed Immunofluorescence Reproducibility Evaluation (MITRE) Study. J Immunother Cancer. 2021 Jul;9(7):e002197. doi: 10.1136/jitc-2020-002197.
- Vanhersecke L, Brunet M, Guégan JP, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. 2021 Aug;2(8):794-802. doi: 10.1038/s43018-021-00232-6. Epub 2021 Aug 12.
- Anadon CM, Yu X, Hänggi K, et al. Ovarian cancer immunogenicity is governed by a narrow subset of progenitor tissue-resident memory T cells. Cancer Cell. 2022 May 9;40(5):545-557.e13. doi: 10.1016/j.ccell.2022.03.008. Epub 2022 Apr 14.
- Attrill GH, Owen CN, Ahmed T, et al. Higher proportions of CD39 tumor-resident cytotoxic T cells predict recurrence-free survival in patients with stage III melanoma treated with adjuvant immunotherapy. J Immunother Cancer. 2022 Jun;10(6):e004771. doi: 10.1136/jitc-2022-004771.
- Melero I, Villalba-Esparza M, Recalde-Zamacona B, et al. Neutrophil Extracellular Traps, Local IL-8 Expression, and Cytotoxic T-Lymphocyte Response in the Lungs of Patients With Fatal COVID-19. Chest. 2022 Nov;162(5):1006-1016. doi: 10.1016/j.chest.2022.06.007. Epub 2022 Jun 15.
- Liu Z, Zhao Y, Kong P, et al. Integrated multi-omics profiling yields a clinically relevant molecular classification for esophageal squamous cell carcinoma. Cancer Cell. 2023 Jan 9;41(1):181-195.e9. doi: 10.1016/j.ccell.2022.12.004. Epub 2022 Dec 29.
- Yang S, Qian L, Li Z, et al. Integrated Multi-Omics Landscape of Liver Metastases. Gastroenterology. 2023 Mar;164(3):407-423.e17. doi: 10.1053/j.gastro.2022.11.029. Epub 2022 Nov 26.








