Stable and consistent immunohistochemical (IHC) staining can reveal the spatial information of biomarkers in tissues and cells, which is crucial in clinical pathology diagnosis, drug development, and translational medicine. However, IHC staining results can vary due to factors such as sample preparation, reagent batches, experimental operations, and the performance of automated instrument platforms. These variations can affect the interpretation and evaluation of the results.
In routine pathological testing, the lack of renewable IHC standards significantly increases medical costs. Compared with IHC, the analytical error rates in clinical chemistry, immunology, and hematology are one order of magnitude lower (<1%).
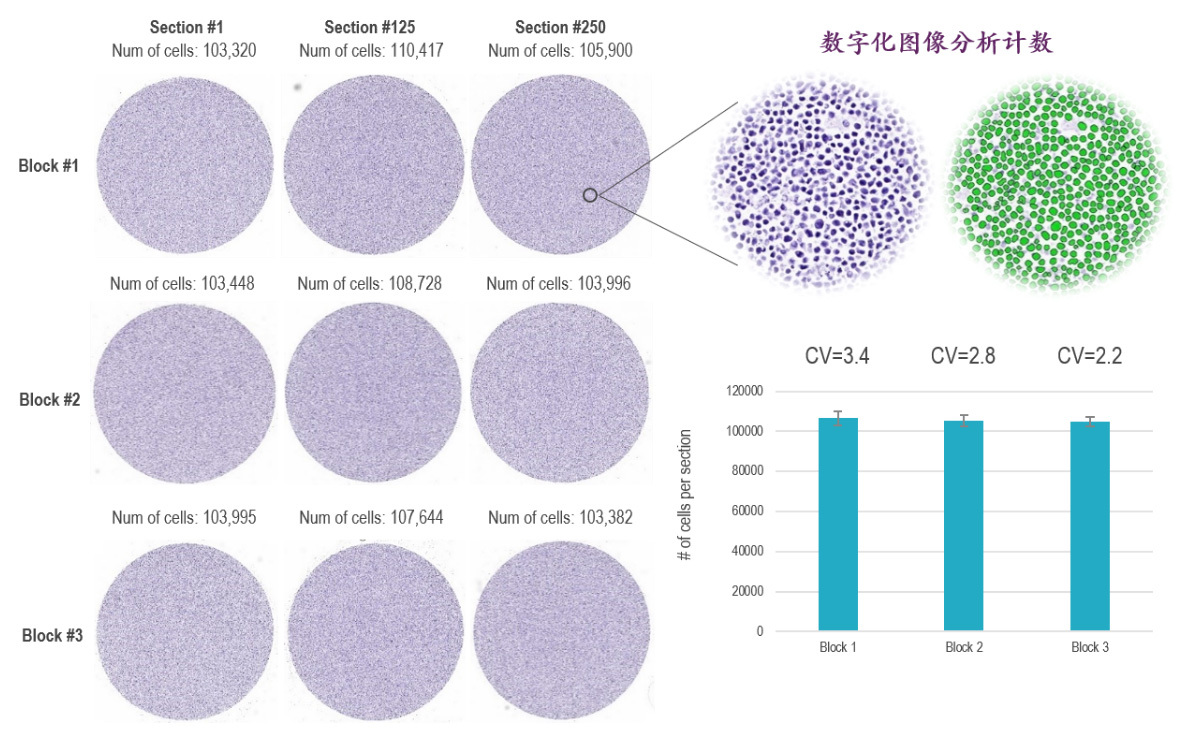
Beijing PhenoVision Biotechnology Co., Ltd. has introduced advanced Cell FFPE Control preparation processes, ensuring a high degree of consistency in cell numbers between paraffin blocks and among each section of a single paraffin block. This enables the company to provide industrial-grade quality control standards for routine pathological testing, such as immunohistochemistry (IHC), in situ hybridization (ISH), and PCR.
1. High Density, High Homogeneity: The process of creating high-density, high-homogeneity cell paraffin blocks and sections ensures consistent staining results between sections and across batches.
2. Controllable Antigen Expression Levels: Pre-prepared cell controls with specific antigen expression levels are available for comparison with tissue samples under the same staining conditions during immunohistochemical (IHC) assays.
3. Cell Array Quality Control System: The cell array quality control system includes cell arrays with positive or graded positive expressions for different antibody targets, which can be used for semi-quantitative or qualitative analysis of staining results.
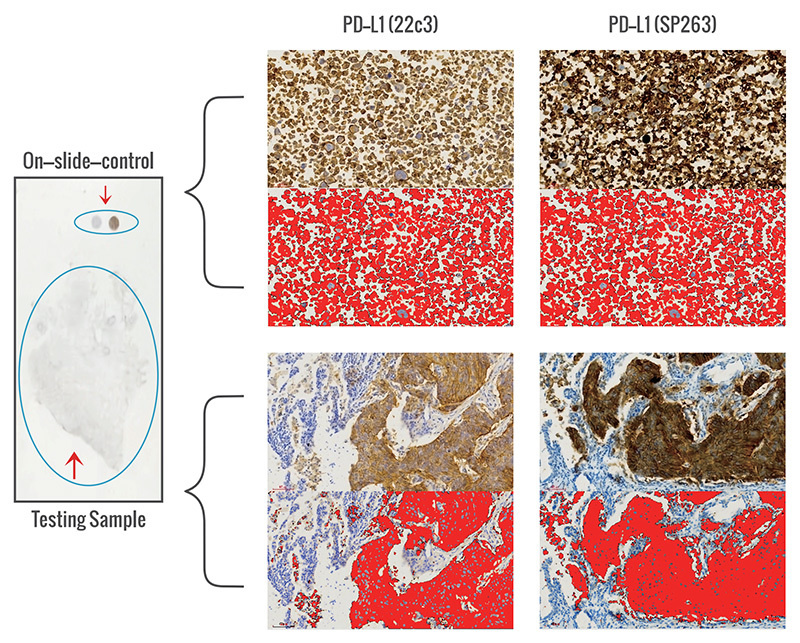
1. Analysis of PD-L1 IHC Clone Differences
A proprietary on-slide-control system for companion diagnostics aids in pathological diagnosis.
Calibration of differences in antibody clones, batch variations of antibodies, dilution concentrations, and experimental procedural variations to address discrepancies in detection results.
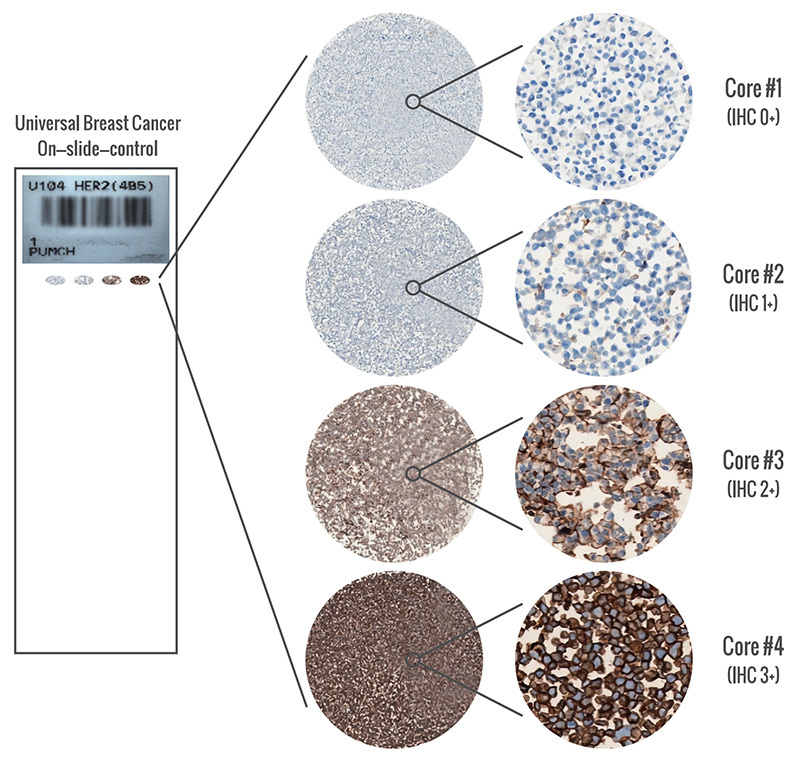
2. HER2 4in1 Tissue Control
It can be used to evaluate information such as the performance of instruments, experimental procedures, reagent quality, and the consistency of experimental results during the HER2 immunohistochemical staining process.